What is the molal concentration of oxygen in water at 20 C that has been saturated with
Question:
What is the molal concentration of oxygen in water at 20 °C that has been saturated with air at 1.00 atm ? Assume that the mole fraction of oxygen in air is 0.21 .
Strategy
Use Henry’s law to calculate the oxygen concentration in the water using the partial pressure of oxygen and the appropriate Henry’s law constant from Table 12.3.
Table 12.3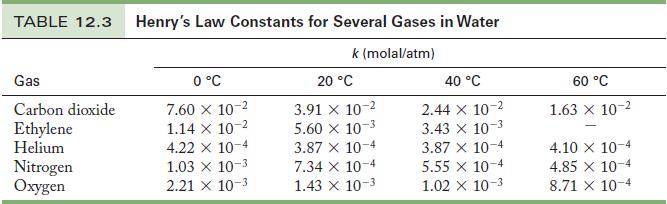
Transcribed Image Text:
TABLE 12.3 Henry's Law Constants for Several Gases in Water k (molal/atm) Gas Carbon dioxide Ethylene Helium Nitrogen Oxygen 0 C 7.60 x 10- 1.14 x 10-2 4.22 x 10-4 1.03 10-3 2.21 x 10-3 20 C 3.91 X 10- 5.60 x 10-3 3.87 x 10-4 7.34 x 10-4 1.43 X 10- 40 C 2.44 x 10- 10-3 3.43 x 3.87 x 10-4 5.55 x 10-4 1.02 X 10-3 60 C 1.63 x 10- 4.10 X 10-4 4.85 x 10-4 8.71 X 10-4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Before applying Henrys law we must fi nd the partial pressure of the oxygen in the g...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
(a) Find a matrix A and a column matrix B that describe the following tables. (b) Find the matrix product AB, and interpret the result. Student 1 Student 2 Student 3 College A College B College C...
-
Produce a flow chart for the following program, use the correct symbols; #include using namespace std; int main() { int i=1; while(i
-
Give the sources of research problem. How a problem is identified? Enumerate the criteria for the selection of a problem.
-
Peppers Lockdown produces keys for homes and cars. As Peppers is planning for next year's production, he decided to implement a high-low system to forecast future costs. With total production of...
-
Find the rms value of the voltage defined by the expression v(t) = cos t + cos (t + 120o)
-
New York Fashions owns 87 womens clothing stores in shopping malls. Corporate headquarters of New York Fashions uses flexible budgets to control the operations of each of the stores. The following...
-
Explain the difference between consent and informed consent.
-
On May 21, 1927, Charles Lindbergh landed at Le Bourget Field in Paris, completing his famous transatlantic solo flight. The preparation period prior to his flight was quite hectic and time was...
-
o Value An economist for a sporting goods company estimates the revenue and cost functions for the production of a new snowboard. These functions are R(x) = -x+ 15x and C(x)=7x+12, respectively,...
-
Straight-chain alcohols [CH 3 (CH 2 )nOH] that contain more than four carbon atoms have limited solubility in water. Predict how the solubility of these alcohols in water changes as the value of n in...
-
What is the molal concentration of nitrogen in this same solution? The mole fraction of nitrogen in air is 0.78.
-
The sulfur dioxide content of a stack gas is monitored by passing a sample stream of the gas through an SO 2 analyzer. The analyzer reading is 1000 ppm 5O 2 (parts per million on a molar basis). The...
-
Evaluate and critically analyze the effect ERP systems have had upon an organization you know well, its operations, and its relationships, using examples to illustrate your points. Include sources.
-
The sections below list the key building blocks of the the of plan for the startup's supply chain. The final plan should reflect the network between the startup and its supplier(s) that enables the...
-
What are some of the key considerations that organizations should keep in mind when selecting an ERP system?How do organizations typically approach the implementation of an ERP system, and what are...
-
Produce a one- to three-page position arguing for or against the statement, "It is better to make a business decision with bad data than with no data." Behind each point of view is the question: (a)...
-
The purpose of this is for you to identify a current influential business leader and: Determine what makes them a "good" leader. Identifies their strategic management process. How they direct the...
-
Your portfolio is comprised of 40 percent of stock X, 15 percent of stock Y, and 45 percent of stock Z. Stock X has a beta of 1.16, stock Y has a beta of 1.47, and stock Z has a beta of 0.42. What is...
-
Starr Co. had sales revenue of $540,000 in 2014. Other items recorded during the year were: Cost of goods sold ..................................................... $330,000 Salaries and wages...
-
Determine (approximately) the force in each member of the truss. Assuming that the diagonals cannot support a compression force. 10 k 10 k 10 k 10 k 5 k 20 ft |B 20 ft- 20 ft- 20 ft-
-
Determine (approximately) the force in each member of the truss. Assume the diagonals can support either a tensile or a compression force. 10 k 10 k 10 k 10 k 20 ft A |C 20 ft- -20 ft- -20 ft-
-
Determine (approximately) the force in each member of the truss. Assume the diagonals can support either a tensile or a compression force. 50 kN 40 kN 20 kN to 3 m A 3 m
-
What does a high PE tell us about the value of the stock price (over or under valued)? What does a low PE tell us about the value of the stock price (over or under valued)? Be specific with your...
-
How can a financial risk can be managed? What is financial risk? what does it include and exclude? What do we study in the financial risk manager?
-
A manufactured product has the following Information for June. Direct materials Direct labor Overhead Units manufactured Standard Quantity and Cost 6 pounds @ $8 per pound 3 DLH @ $17 per DLH 3 DLH @...

Study smarter with the SolutionInn App


