What mass of water forms when oxygen gas in the container below, where each red molecule represents
Question:
What mass of water forms when oxygen gas in the container below, where each red molecule represents 0.10 of a mole, reacts with hydrogen gas in the other container, where each white molecule represents 0.10 of a mole.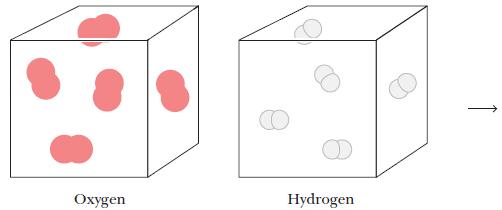
Transcribed Image Text:
Oxygen Hydrogen
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
5 x 010 mol oxygen 5 x 010 mol hydrogen mass ...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
A frictionless piston of mass m is a precise fit in the vertical cylindrical neck of a large container of volume V. the container is filled with a gas and there is a vacuum above the piston. The...
-
A gaseous material XY(g) dissociates to some extent to produce X(g) and Y(g): XY(g) X(g) + Y(g) A 2.00- g sample of XY (molar mass = 165 g/ mol) is placed in a container with a movable piston at...
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
State the date (or dates) on which corporation tax is due for payment in relation to the following periods of account: (a) the year to 31 March 2021 (b) the six months to 30 November 2020 (c) the 21...
-
Explain in terms of molecular motion why the pressure on the walls of a container increases when the volume of a gas is reduced at constant temperature.
-
A small object of mass m carries a charge q and is suspended by a thread between the vertical plates of a parallel-plate capacitor. The plate separation is d. If the thread makes an angle with the...
-
Prepare headings for a cash disbursements journal like the one in Exhibit E-A.4. Journalize the April transactions from Exercise E-10 that should be recorded in the cash disbursements journal...
-
Shaw is a lumber company that also manufactures custom cabinetry. It is made up of two divisions: Lumber and Cabinetry. The Lumber Division is responsible for harvesting and preparing lumber for use;...
-
As a practical matter, variances from standard are usually: A. closed to Manufacturing Overhead. B. closed directly to Income Summary. C. allocated to Work in Process Inventory, Finished Goods...
-
Vincent Cardoza is the owner and manager of a machine shop that does custom order work. This Wednesday afternoon, he has received calls from two customers who would like to place rush orders. One is...
-
What is the partial pressure of argon, in torr, in a container that also contains neon at 235 torr and is at a total pressure of 500 torr?
-
What is the total pressure, in atm, in a container that holds 1.22 atm of hydrogen gas and 4.33 atm of argon gas?
-
Given the assumptions in column 1 of the table, show that the assumptions in column 2 are equivalent to them. Assumptions of the Classical Model (1).................... (2) E(u i \X i )...
-
10.) Steam enters a well-insulated turbine at 6 MPa, 400C and expands to 200 kPa, saturated vapor at a rate of 10 kg/s. (a) Draw a schematic of the process (5 pts). (b) Determine the exergy...
-
4. [8 marks] The tides in the Bay of Fundy are some of the largest in the world. The height, h(t), of the tide in meters after t hourse can be modeled by 39 h(t) = 25 con (77) + 30 4 COS 6 (a) What...
-
Wolfe, Inc. had credit sales for the period of $144,000. The balance in Allowance for Doubtful Accounts is a debit of $653. If Wolfe estimates that 2% of credit sales will be uncollectible, what is...
-
Water at 20C is to be pumped from a reservoir (ZA = 5 m) to another reservoir at a higher elevation (ZB = 13 m) through two 36-m- long pipes connected in parallel as shown. The pipes are made of...
-
Delph Company uses a job-order costing system with a plantwide predetermined overhead rate based on machine-hours. At the beginning of the year, the company estimated that 53,000 machine-hours would...
-
Consider a Pb-15% Sn alloy. During solidification, determine (a) The composition of the first solid to form; (b) The liquids temperature, solidus temperature, solvus temperature, and freezing range...
-
Starr Co. had sales revenue of $540,000 in 2014. Other items recorded during the year were: Cost of goods sold ..................................................... $330,000 Salaries and wages...
-
Use the anharmonic potential function in Figure 18.7 to demonstrate that rotation and vibration are not separable degrees of freedom for large quantum numbers. Figure 18.7 (X)A
-
Conservation of energy requires that the variation of the potential and kinetic energies with the oscillator extension be exactly out of phase. Explain this statement.
-
What is the degeneracy of the energy levels for the rigid rotor in two dimensions? If it is not 1, explain why.
-
Production numbers for 2 shifts are shown. The shift supervisor of Shift 2 insists to the production manager that her operators are more productive than the ones on Shift 1. Using a confidence level...
-
In a class, the scores that students got are as shown. What are the 25, 50, 75 and 100th percentiles for the data? 84 84 98 80 89 83 85 56 85 84 84 74 84 81 83 80 45 86 67 79 81 78 76 85 83 77 86 83...
-
Number of points made by Teams A and B are shown. Which statement is true based on running the F-Test Two-Sample for Variances in the Data Analysis pack in Excel? Use a confidence level of 10% to...

Study smarter with the SolutionInn App


