Write the molecular orbital diagram and the electron configuration for (a) Be 2 (b) B
Question:
Write the molecular orbital diagram and the electron configuration for
(a) Be2
(b) B2.
Predict the bond order and the number of unpaired electrons for each molecule.
Strategy
Use the diagram in Figure 10.40, adding electrons as appropriate to the lowest energy orbitals.
Figure 10.40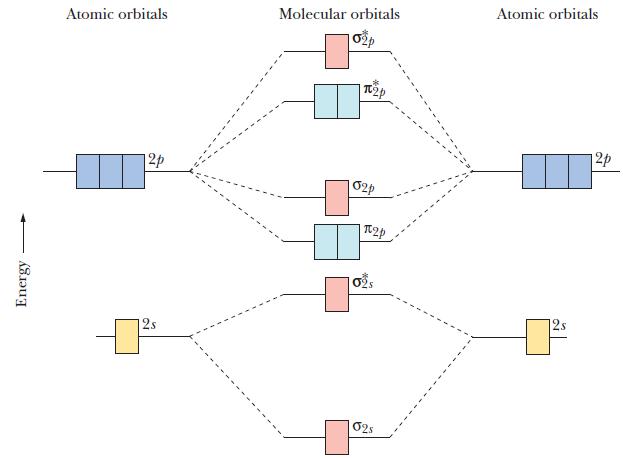
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





