The pH scale is used to measure the acidity or alkalinity of a solution. The scale ranges
Question:
The pH scale is used to measure the acidity or alkalinity of a solution. The scale ranges from 0 to 14. A neutral solution, such as pure water, has a pH of 7. An acid solution has a pH less than 7 and an alkaline solution has a pH greater than 7. The lower the pH below 7, the more acidic is the solution. Each whole-number decrease in pH represents a tenfold increase in acidity.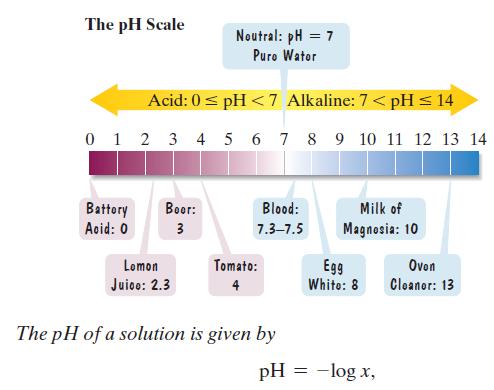 where x represents the concentration of the hydrogen ions in the solution, in moles per liter. Use the formula to solve Exercises 119–120. Express answers as powers of 10.
where x represents the concentration of the hydrogen ions in the solution, in moles per liter. Use the formula to solve Exercises 119–120. Express answers as powers of 10.
a. Normal, unpolluted rain has a pH of about 5.6. What is the hydrogen ion concentration?
b. An environmental concern involves the destructive effects of acid rain. The most acidic rainfall ever had a pH of 2.4. What was the hydrogen ion concentration?
c. How many times greater is the hydrogen ion concentration of the acidic rainfall in part (b) than the normal rainfall in part (a)?
Step by Step Answer:






