Question: As covered in the Process Description, there are three primary reactions that occur in the MSR. These are given by Equations 13.3, 13.4, and 13.5.
As covered in the Process Description, there are three primary reactions that occur in the MSR. These are given by Equations 13.3, 13.4, and 13.5. However, determination of chemical equilibrium among the species H2, CO, CO2, H2O, and CH3OH involves only two of the three reactions because each reaction is a linear combination of the other two. Cherednichenko gives an approximation for the equilibrium relationships in Equation 13.3 (see Problem 13.13) and in Equation 13.5:
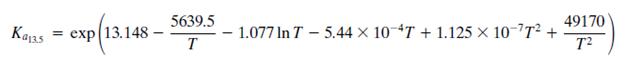
The equilibrium Ka13.5 constant is defined by the relationship
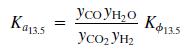
Where T is in kelvin and Kϕ13.5 accounts for nonideal behavior of the gas phase.
a. As in Problems 13.13 and 13.14, suppose the synthesis gas leaving the MUG compressor is fed directly to theMSRand that the composition of this gas is 5 mole% methane, 25%CO, 5%CO2, and the remainder hydrogen. The product stream leaving the MSR is at 250°C and 7.5 MPa and you may assume Kϕ13.3 = Kϕ13.5 = 0. Again determine the conversions of CO and H2 with this process configuration, and use these results to justify utilization of the recycle loop.
b. Show that removal of water from the reformer product gas minimizes the impact on conversion of CO to methanol by determining the effect on CO and H2 conversions and selectivity as defined by moles CH3OH formed per mole of CO reacted if the given composition of MSR feed gas in part (a) is on a dry basis and the stream itself contained 5 mole% water.
Equations 13.3, 13.4, and 13.5
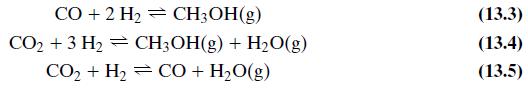
5639.5 49170 Karas exp(13.148 - T 1.077 In T - 5.44 x 10T + 1.125 x 10-7T2 +
Step by Step Solution
3.56 Rating (160 Votes )
There are 3 Steps involved in it
a Using Equation 133 and assuming K133 K135 0 the conve... View full answer

Get step-by-step solutions from verified subject matter experts


