Question: With sufficient energy, it?s possible to eject an electron from an inner atomic orbital. A higher-energy electron will then drop into the unoccupied state, emitting
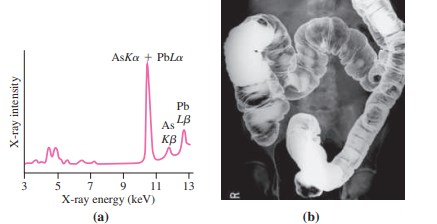
With sufficient energy, it?s possible to eject an electron from an inner atomic orbital. A higher-energy electron will then drop into the unoccupied state, emitting a photon with energy equal to the difference between the two levels. For inner-shell electrons, photon energies are in the keV range, putting them in the X-ray region of the spectrum. These characteristic X rays are labeled with the letter indicating the shell to which the electron drops, followed by a Greek letter indicating the higher level from which it drops; thus Ka designates a transition from the L shell to the K shell. Characteristic X rays provide scientists and physicians with an important diagnostic tool. Environmental scientists bombard pollution samples with high-energy electrons, knocking out inner-shell electrons and thus producing X-ray spectra that help identify contaminants (Fig. 36.20a). Geologists do the same with rocks. Medical radiologists reverse the process, exploiting the fact that X rays cause inner-shell transitions as well as complete ejection of inner-shell electrons. In particular, radiologists use the element barium in this way to produce high-contrast X-ray images of the intestinal tract (Fig. 36.20b).
?
Emission of characteristic X rays occurs in the context of multielectron atoms that generally have all but one of their electrons present. You should therefore expect the X-ray energies to be describeda. quite accurately by Bohr?s atomic theory.b. through hydrogen-like solutions to the Schr?dinger equation.c. only approximately by Bohr?s theory or hydrogenic solutions to the Schr?dinger equation.
AsKa + PbLa Pb As 48 KB A 5 7 X-ray energy (keV) 9 11 13 (a) (b) X-ray intensity R.
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it
C only approximately by Bohrs theory or hydrogenic solutions to the Schrdinger equation T... View full answer

Get step-by-step solutions from verified subject matter experts


