Question: Consider a graph of the half cell potentials in Table 8.2 vs. the density of the electrode metal in the half cell. Explain whether or
Consider a graph of the half cell potentials in Table 8.2 vs. the density of the electrode metal in the half cell. Explain whether or not it is possible to design a cell that is both light and high voltage (say, more than 3 V).
Table 8.2
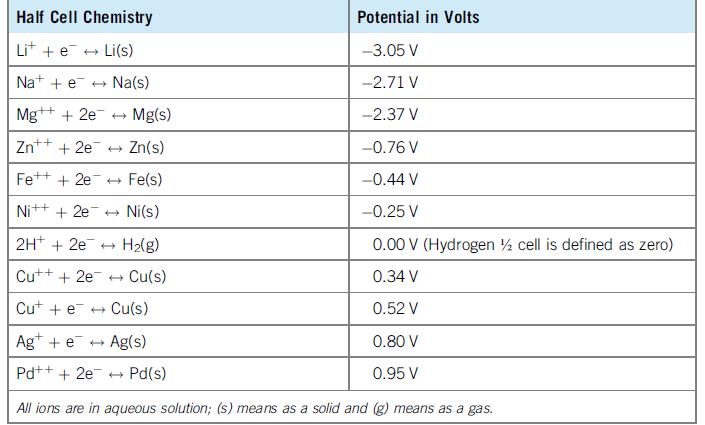
Half Cell Chemistry Potential in Volts Lit + e + Li(s) -3.05 V Nat + e + Na(s) -2.71 V Mg++ + 2e + Mg(s) -2.37 V Zn* ++ + 2e + Zn(s) -0.76 V Fe++ + 2e + Fe(s) -0.44 V Nit+ + 2e + Ni(s) -0.25 V 2H* + 2e + H2(g) 0.00 V (Hydrogen % cell is defined as zero) Cut+ + 2e + Cu(s) 0.34 V Cut + e + Cu(s) 0.52 V Ag+ + e + Ag(s) 0.80 V Pd++ + 2e + Pd(s) 0.95 V All ions are in aqueous solution; (s) means as a solid and (g) means as a gas.
Step by Step Solution
3.38 Rating (154 Votes )
There are 3 Steps involved in it
HighVoltage Materials Materials with higher positive potentials include Ag Pd and Cu These ... View full answer

Get step-by-step solutions from verified subject matter experts


