Mercury(II) forms a 1:1 complex with triphenyltetrazolium chloride (TTC) that exhibits an absorption maximum at 255 nm.
Question:
Mercury(II) forms a 1:1 complex with triphenyltetrazolium chloride (TTC) that exhibits an absorption maximum at 255 nm. The mercury(II) in a soil sample was extracted into an organic solvent containing an excess of TTC, and the resulting solution was diluted to 100.0 mL in a volumetric flask. Five-milliliter aliquots of the analyte solution were then transferred to six 25-mL volumetric flasks. A standard solution was then prepared that was 5.00 x 10-6 M in Hg(II). Volumes of the standard solution shown in the table were then pipetted into the volumetric flasks, and each solution was then diluted to 25.00 mL. The absorbance of each solution was measured at 255 nm in 1.00-cm quartz cells.
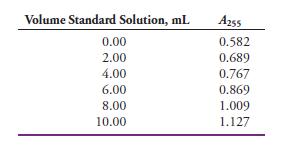
(a) Enter the data into a spreadsheet, and construct a standard additions plot.
(b) Determine the slope and intercept of the line.
(c) Determine the standard deviation of the slope and of the intercept.
(d) Calculate the concentration of Hg(II) in the analyte solution.
(e) Find the standard deviation of the measured concentration.
Step by Step Answer:

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch





