The chromium in a series of steel samples was determined by ICP emission spectroscopy. The spectrometer was
Question:
The chromium in a series of steel samples was determined by ICP emission spectroscopy. The spectrometer was calibrated with a series of standards containing 0, 2.0, 4.0, 6.0, and 8.0 μg K2Cr2O7 per milliliter. The instrument readings for these solutions were 3.1, 21.5, 40.9, 57.1, and 77.3 in arbitrary units.
(a) Plot the data.
(b) Find the equation for the regression line.
(c) Calculate standard deviations for the slope and the intercept of the line in (b).
(d) The following data were obtained for replicate 1.00-g samples of cement dissolved in HCl and diluted to 100.0 mL after neutralization:
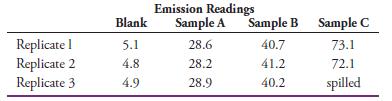
Calculate the percent Cr2O3 in each sample. What are the absolute and relative standard deviations for the average of each determination?
Step by Step Answer:

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch





