The indicator HIn has an acid dissociation constant of 4.80 x 10 -6 at ordinary temperatures. The
Question:
The indicator HIn has an acid dissociation constant of 4.80 x 10-6 at ordinary temperatures. The accompanying absorbance data are for 8.00 x 10-5 M solutions of the indicator measured in 1.00-cm cells in strongly acidic and strongly alkaline media:
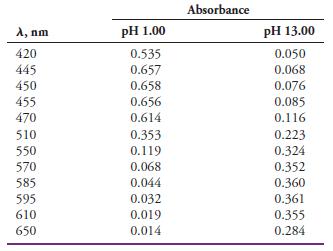
Estimate the wavelength at which absorption by the indicator becomes independent of pH (that is, the isosbestic point).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
Question Posted:





