It is desired to diffuse a blue dye molecule (solute A) into a solid material (species B)
Question:
It is desired to diffuse a blue dye molecule (solute A) into a solid material (species B) near the surface of the material, as shown in the figure below. Initially, there is no dye in the solid (cAo = 0), and the maximum solubility of the dye in the solid is 0.010 gmole/m3. Both the solubility of the dye and its diffusion coefficient in the solid are relatively small, so that the dye does not penetrate very far into the solid. The process is carried out at ambient conditions (25 C, 1.0 atm). After 2.0 min (120 s), the flux of the dye into the solid was observed to be NA(0,t) 5.15 x 10-9 gmole/m2s.
a. The process is considered complete when the dye concentration within the solid achieves a concentration of 0.0062 gmole/m3 at a depth of 0.050 cm from the surface. How much time will this process take?
b. Which of the following are true about solid B: transient source, transient sink, stagnant medium, semi-infinite medium?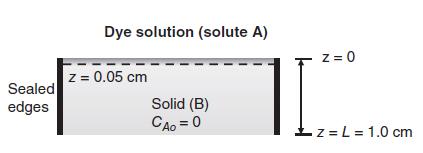
Step by Step Answer:

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781119723547
7th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster





