The boiling temperature of nitrogen at atmospheric pressure at sea level (1 atm pressure) is 196
Question:
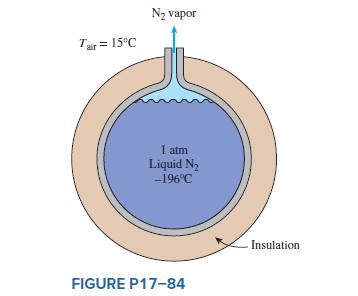
The boiling temperature of nitrogen at atmospheric pressure at sea level (1 atm pressure) is − 196° C. Therefore, nitrogen is commonly used in low-temperature scientific studies since the temperature of liquid nitrogen in a tank open to the atmosphere will remain constant at − 196° C until it is depleted. Any heat transfer to the tank will result in the evaporation of some liquid nitrogen, which has a heat of vaporization of 198 kJ/kg and a density of 810 kg/ m3 at 1 atm.
Consider a 3-m-diameter spherical tank that is initially filled with liquid nitrogen at 1 atm and − 196° C. The tank is exposed to ambient air at 15° C, with a combined convection and radiation heat transfer coefficient of 35 W/ m2⋅K. The temperature of the thin-shelled spherical tank is observed to be almost the same as the temperature of the nitrogen inside. Determine the rate of evaporation of the liquid nitrogen in the tank as a result of the heat transfer from the ambient air if the tank is (a) not insulated, (b) insulated with 5-cm-thick fiberglass insulation (k = 0.035 W/m⋅K), and (c) insulated with 2-cm-thick superinsulation which has an effective thermal conductivity of 0.00005 W/m⋅K.
Step by Step Answer:

Fundamentals Of Thermal-Fluid Sciences
ISBN: 9781260716979
6th Edition
Authors: Yunus Cengel, John Cimbala, Afshin Ghajar





