A piston/cylinder contains 0.5 kg of water at 200 kPa, 300C, and it now cools to 150C
Question:
A piston/cylinder contains 0.5 kg of water at 200 kPa, 300◦C, and it now cools to 150◦C in an isobaric process. The heat goes into a heat engine that rejects heat to the ambient at 25◦C (shown in Fig. P6.46), and the whole process is assumed to be reversible. Find the heat transfer out of the water and the work given out by the heat engine.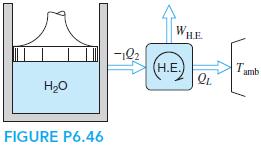
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:





