A piston/cylinder contains helium at 110 kPa at ambient temperature 20C and an initial volume of 20
Question:
A piston/cylinder contains helium at 110 kPa at ambient temperature 20◦C and an initial volume of 20 L, as shown in Fig. P11.136. The stops are mounted to give a maximum volume of 25 L, and the nitrogen line conditions are 300 kPa, 30◦C. The value is now opened, which allow nitrogen to flow in and mix with the helium. The valve is closed when the pressure inside reaches 200 kPa, at which point the temperature inside is 40◦C. Is this process consistent with the second law of thermodynamics? 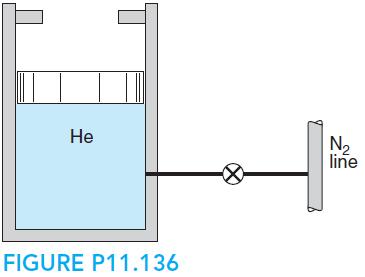
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:





