Solid carbon is burned with stoichiometric air in a steady-flow process. The reactants at T 0 ,
Question:
Solid carbon is burned with stoichiometric air in a steady-flow process. The reactants at T0, P0 are heated in a preheater to T2 = 500 K, as shown in Fig. P13.97, with the energy given by the product gases before flowing to a second heat exchanger, which they leave at T0. Find the temperature of the products T4 and the heat transfer per k mol of fuel (4 to 5) in the second heat exchanger.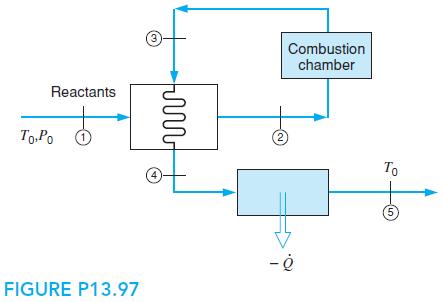
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:





