Question: Water is used as the working fluid in a Carnot-cycle heat engine, where it changes from saturated liquid to saturated vapor at 200C as heat
Water is used as the working fluid in a Carnot-cycle heat engine, where it changes from saturated liquid to saturated vapor at 200◦C as heat is added. Heat is rejected in a constant-pressure process (also constant T) at 20 kPa. The heat engine powers a Carnot cycle refrigerator that operates between−15◦C and +20◦C, shown in Fig. P6.38. Find the heat added to the water per kilogram of water. How much heat should be added to the water in the heat engine so that the refrigerator can remove 1 kJ from the cold space?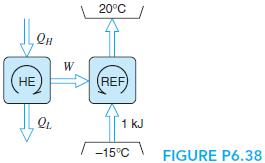
20C W (REF) 1 kJ -15C FIGURE P6.38
Step by Step Solution
3.36 Rating (162 Votes )
There are 3 Steps involved in it
The heat added to the water per kilogram of water is 34 kJkg The heat that shoul... View full answer

Get step-by-step solutions from verified subject matter experts


