An alloy of aluminum and magnesium was treated with sodium hydroxide solution, in which only aluminum reacts.
Question:
An alloy of aluminum and magnesium was treated with sodium hydroxide solution, in which only aluminum reacts.
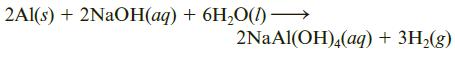
If a sample of alloy weighing 1.225 g gave 0.1093 g of hydrogen, what is the percentage of aluminum in the alloy?
4.158 An alloy of iron and carbon was treated with sulfuric acid, in which only iron reacts.

If a sample of alloy weighing 2.358 g gave 0.1067 g of hydrogen, what is the percentage of iron in the alloy?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





