Carbon disulfide, CS 2 , burns in oxygen. Complete combustion gives the balanced reaction that has been
Question:
Carbon disulfide, CS2, burns in oxygen. Complete combustion gives the balanced reaction that has been depicted here using molecular models.
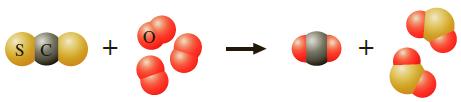
Calculate the grams of sulfur dioxide, SO2, produced when a mixture of 35.0 g of carbon disulfide and 35.0 g of oxygen reacts. Which reactant remains unconsumed at the end of the combustion? How many grams remain?
Transcribed Image Text:
S C + +
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
First determine whether CS 2 or O 2 is the limiting reactant by calcu...View the full answer

Answered By

Rinki Devi
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions.
Hi there! Are you looking for a committed, reliable, and enthusiastic tutor? Well, teaching and learning are more of a second nature to me, having been raised by parents who are both teachers. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students.
I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and helped them achieve great subject knowledge.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Carbon disulfide, CS2, burns in oxygen. Complete combustion gives the balanced reaction that has been depicted here using molecular models. Calculate the grams of sulfur dioxide, SO2, produced when a...
-
Carbon disulfide burns in air, producing carbon dioxide and sulfur dioxide. CS2(l) + 3O2(g) CO2(g) + 2SO2(g); H = 1077 kJ What is H for the following equation? ics2(1) + 302(g)--CO2(g) + SO2(g) CS2
-
Carbon disulfide (CS2) is a toxic, highly flammable substance. The following thermodynamic data are available for CS2(l) and CS2(g) at 298 K: (a) Draw the Lewis structure of the molecule. What do you...
-
Consider the approximation of the welfare loss due to inter-area deviations from the correct rate of care. All else equal, which procedures would yield the largest welfare losses those with low price...
-
In Exercises 19-22, match the equation with one of the graphs (a)-(d), which follow. a. b. c. d. 1. 16x2 + 4y2 = 64 2. 4x2 + 5y2 = 20 3. x2 + 9y2 - 6x + 90y = -225 4. 9x2 + 4y2 + 18x - 16y = 11...
-
Explain how a client process finds the IP address and the port number to be inserted in a remote socket address.
-
A hypodermic syringe (see Fig. P5.30) is used to apply a vaccine. If the plunger is moved forward at the steady rate of \(20 \mathrm{~mm} / \mathrm{s}\) and if vaccine leaks past the plunger at 0.1...
-
CMA Review Inc. provides review courses for students studying to take the CMA exam. The cost of textbooks is included in the registration fee. Text material requires constant updating and is useful...
-
Discuss why 7 . A four - stroke cycle engine may or may not have a pressure boost in the intake system. 8 . The two - stroke cycle engine must always have an intake pressure boost. 9 . The marine...
-
Suppose we have an economy with two consumers, i = 1, 2, who have identical Cobb- Douglas preferences over their private goods consumption, xi, and a public good, g, that can be represented by the...
-
Methyl salicylate (oil of wintergreen) is prepared by heating salicylic acid, C 7 H 6 O 3 , with methanol, CH 3 OH. C 7 H 6 O 3 + CH 3 OH C 8 H 8 O 3 + H 2 O In an experiment, 2.50 g of salicylic...
-
Solutions of sodium hypochlorite, NaClO, are sold as a bleach (such as Clorox). They are prepared by the reaction of chlorine with sodium hydroxide. 2NaOH(aq) + Cl 2 (g) NaCl(aq) + NaClO(aq) + H 2...
-
What does the code for an empty dictionary look like?
-
How does implementing Total Quality Management (TQM) contribute to enhancing both product and service quality within an organization?
-
Mr. Jorgensen, a shareholder in the Best Corporation, owns 200 shares of ts common stock. Mr. Jorgensen receives a 7% stock dividend, After the stock dividend, Mit Jorgensen will have a: O total of...
-
A 170-N solid sphere 0.20 m in radius rolls without slipping 6.0 m down a ramp that is inclined at 34 with the horizontal. What is the angular speed of the sphere at the bottom of the slope if it...
-
How does a firm's external environment impact its strategic decision making process?
-
What are some best practices for writing clean and maintainable code? Provide examples of code snippets demonstrating these practices.
-
In 2015, Bertha Jarow (head of household with three dependents) had a $28,000 loss from the sale of a personal residence. She also purchased from an individual inventor for $7,000 (and resold in two...
-
You've been asked to take over leadership of a group of paralegals that once had a reputation for being a tight-knit, supportive team, but you quickly figure out that this team is in danger of...
-
A 3.0-L sample of paint that has a density of 4.65 g/mL is found to contain 27.5 g Pb3N2(s). How many grams of lead were in the paint sample?
-
A 12.1-g sample of Na2SO3 is mixed with a 15.5-g sample of MgSO4. What is the total mass of oxygen present in the mixture?
-
Potassium superoxide, KO2, is employed in a selfcontained breathing apparatus used by emergency personnel as a source of oxygen. The reaction is 4KO2(s) + 2H2O(l) 4KOH(s) + 3O2(g) Say a...
-
Write a function that takes in a value x, a value el, and a list and adds as many el's to the end of the list as there are x's in the list. Make sure to modify the original list using list mutation...
-
Our office building has a total square footage of 120,000 square feet.We have 9 tenants in the building and no vacancies.The total square footage of the tenant spaces is 105,000.A) What is the Load...
-
00 00 1) A state transition diagram for a synchronous finite state machine is shown in Figure 3. Derive the combinatorial logic required to implement this FSM with the use of Karnaugh maps. Note that...

Study smarter with the SolutionInn App


