Draw a figure similar to Figure 9.2 but for the energetics of ionic bonding in KF. Use
Question:
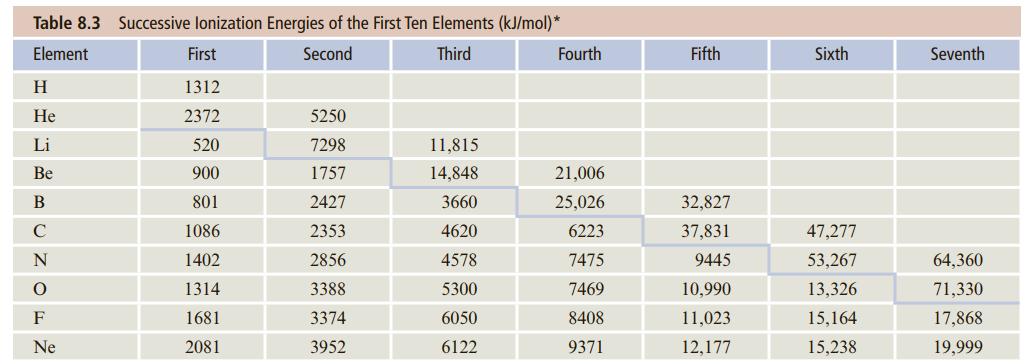
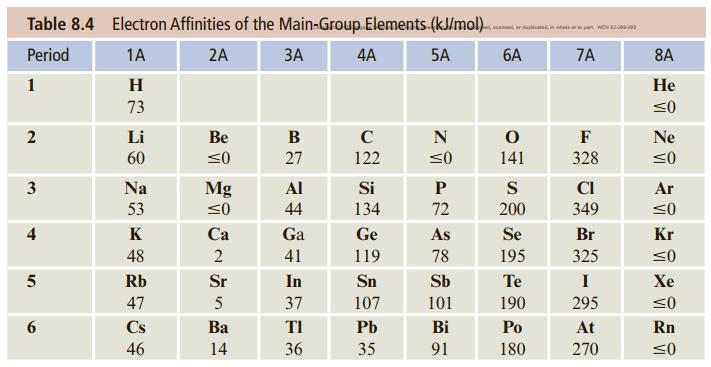
Draw a figure similar to Figure 9.2 but for the energetics of ionic bonding in KF. Use the ionization energy and electron affinity from values given in Tables 8.3 and 8.4 in Chapter 8. Calculate the sublimation energy from thermodynamic data (Appendix C). For the lattice energy, see Problem 9.118.
Figure 9.2
Problem 9.118
Calculate the lattice energy of potassium fluoride, KF, using the Born–Haber cycle. Use thermodynamic data from Appendix C to obtain the enthalpy changes for each step.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





