While roaming a parallel universe, you discover the hypothetical element Z. You obtain a representative sample of
Question:
While roaming a parallel universe, you discover the hypothetical element “Z.” You obtain a representative sample of the element and discover that it is made up of two isotopes, Z-47 and Z-51. To help your science team calculate the atomic weight of the substance, you send the following drawing of your sample with your report.
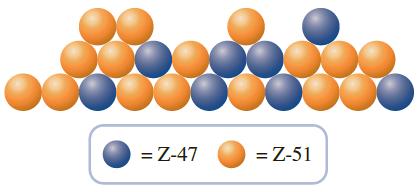
In the report, you also inform the science team that the blue atoms are Z-47, which have an isotopic mass of 47.621 amu, and the orange atoms are Z-51, which have an isotopic mass of 51.217 amu. What is the atomic weight of element Z?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





