(a) Equation 14.47 shows the reaction of Na with CCl 4 . From the following data, confirm...
Question:
(a) Equation 14.47 shows the reaction of Na with CCl4. From the following data, confirm the value of ΔrGº = –1478 kJ mol−1 at 298 K. Data: ΔfG°(NaCl, s) = –384 kJ mol−1; ΔfHº (CCl4, l) = –128.4 kJ mol−1; Sº(CCl4, l) = 214 JK−1 mol−1; Sº(C, gr) = +5.6 JK−1 mol−1; Sº(Cl2, g) = 223 JK−1 mol−1.
(b) Comment on similarities and differences between the structures of b-cristobalite and a noncrystalline silica glass.
Equation 14.47
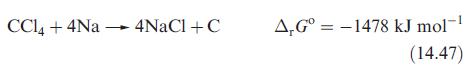
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





