(a) Give three examples of commonly used ionic liquids. What general properties make ionic liquids attractive in...
Question:
(a) Give three examples of commonly used ionic liquids. What general properties make ionic liquids attractive in ‘green chemistry’? Are the properties of the liquid itself all that determines whether the ionic liquid is environmentally friendly?
(b) The compound shown below was treated with one equivalent of NaOH in methanol to give the ionic liquid X:
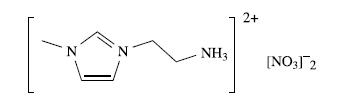
The positive mode electrospray mass spectrum of X exhibits a peak at m/z 126. Compound X reacts with chloroauric acid, HAuCl4, to give a salt, Y, which contains two square planar Au(III) environments. The anion in Y has D4h symmetry. The 1H NMR spectrum of a DMSO-d6 solution of Y exhibits the following signals: δ/ ppm 9.07 (s, 1H), 7.92 (br,2H), 7.74 (overlapping signals, 2H), 4.38 (t, 2H), 3.86 (s, 3H), 3.34 (m, 2H). The electrospray mass spectrum of Y shows peak envelopes at m/z 428 and 126 (positive mode), and 339 (negative mode). The peak envelope at m/z 339 consists of four peaks, and that at m/z 428 exhibits three dominant peaks at m/z 428, 429 and 432. Identify X and Y, and interpret the data. Rationalize the appearance of the mass spectra.
Step by Step Answer:






