(a) Use the values of E for reactions 16.32 and 16.33 to show that H 2 O...
Question:
(a) Use the values of Eº for reactions 16.32 and 16.33 to show that H2O2 is thermodynamically unstable with respect to decomposition into H2O and O2.
(b) ‘20 Volume’ H2O2 is so called because 1 volume of the solution liberates 20 volumes of O2 when it decomposes. If the volumes are measured at 273K and 1 bar pressure, what is the concentration of the solution expressed in grams of H2O2 per dm3?
Equations
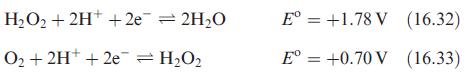
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





