Question: Consider a trigonal prismatic six-coordinate ML 6 complex with D 3h symmetry. Use the D 3h character table (Resources section 4) to divide the d
Consider a trigonal prismatic six-coordinate ML6 complex with D3h symmetry. Use the D3h character table (Resources section 4) to divide the d orbitals of the metal into sets of defined symmetry type. Assume that the ligands are at the same angle relative to the xy-plane as in a tetrahedral complex.
Data from Resource section 4.
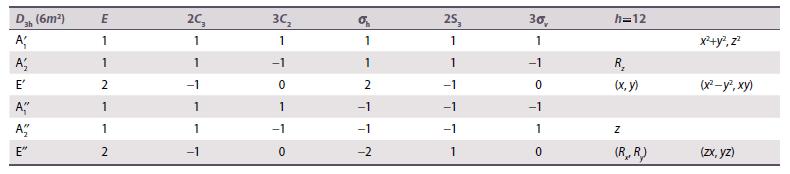
D3h (6m) A A E' A A E" E 1 1 2 1 1 2 2C 1 1 -1 1 -1 30 1 -1 1 -1 0 1 1 2 -1 -1 -2 25, 1 1 -1 -1 1 30, 1 -1 0 -1 1 0 h=12 R (x, y) Z (R,,R) x+y2, z (x-y, xy) (zx, yz)
Step by Step Solution
3.42 Rating (161 Votes )
There are 3 Steps involved in it
To divide the d orbitals into sets of defined symmetry type for a trigonal prismatic sixcoordinate M... View full answer

Get step-by-step solutions from verified subject matter experts


