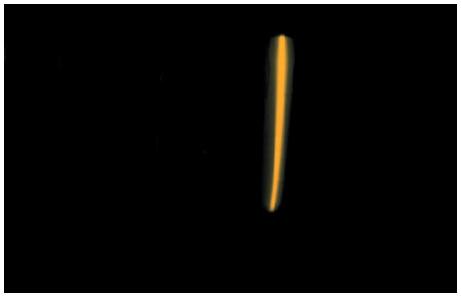
Figure 1.18 shows the emission spectrum of sodium. Low-pressure sodium street lamps depend upon this bright yellow
Question:
Figure 1.18 shows the emission spectrum of sodium. Low-pressure sodium street lamps depend upon this bright yellow emission from sodium atoms excited by an electrical discharge. Figure 1.18 shows a simple spectrum (see figure caption), but the National Institute of Standards and Technology (NIST) atomic spectra database lists 5888 lines in the emission spectrum of sodium.
(a) Suggest three reasons why no other lines are visible in Fig. 1.18.
(b) The wavelengths of the yellow lines in Fig. 1.18 are close to 589 nm. To what frequency does this correspond?
(c) Give a general explanation of how a series of spectral lines such as those in Fig. 1.18 arises
Figure 1.18
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





