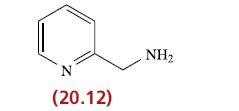
Question: Ligand 20.12 forms an octahedral complex, [Fe(20.12) 3 ] 2+ . (a) Draw diagrams to show what isomers are possible. (b) [Fe(20.12) 3 ]Cl 2
Ligand 20.12 forms an octahedral complex, [Fe(20.12)3]2+.
(a) Draw diagrams to show what isomers are possible.
(b) [Fe(20.12)3]Cl2 exhibits spin crossover at 120 K. Explain clearly what this statement means.
N (20.12) NH
Step by Step Solution
3.48 Rating (161 Votes )
There are 3 Steps involved in it
a When we consider an octahedral complex Fe2012 it is important to identify whether the ligand 2012 can form different isomers by binding in distinct ... View full answer

Get step-by-step solutions from verified subject matter experts


