Resonance structures for urea are represented below: The IR spectrum of free urea has absorptions at 3500
Question:
Resonance structures for urea are represented below:
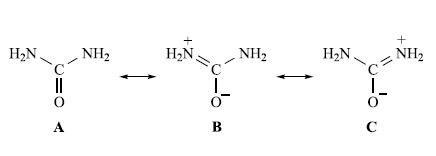
The IR spectrum of free urea has absorptions at 3500 and 3350 (ν(NH2)), 1683 (ν(CO)) and 1471 cm−1 (ν(CN)). Urea can bond to metal ions through either an N- or O-donor atom. When urea bonds through the O atom, the contribution from resonance form A decreases. In the IR spectrum of [Pt(urea)6]Cl2, bands at 3390, 3290, 3130, 3030, 1725 and 1395 cm−1 are assigned to the vibrational modes of metal-bound urea. Suggest why these data suggest the formation of Pt–N rather than Pt–O bonds.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





