Question: Using the data in Table 13.1, draw a potential diagram for Tl and determine the value of E(Tl 3+ /Tl + ). Table 13.1 Property
Using the data in Table 13.1, draw a potential diagram for Tl and determine the value of Eº(Tl3+/Tl+).
Table 13.1
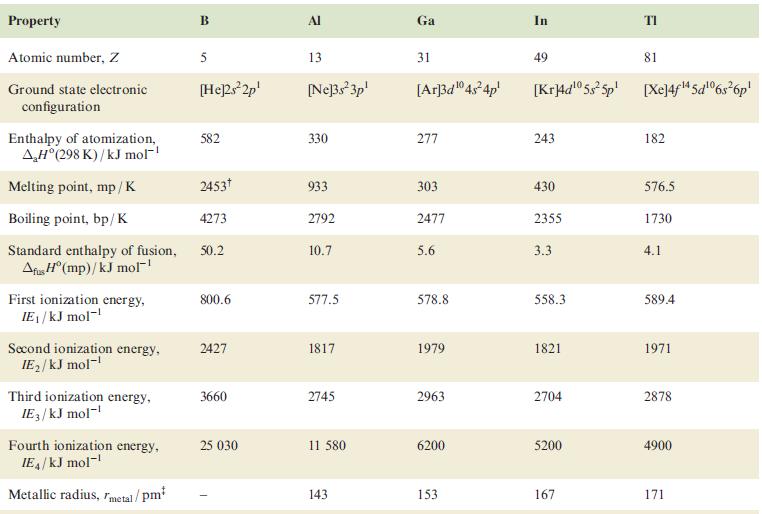
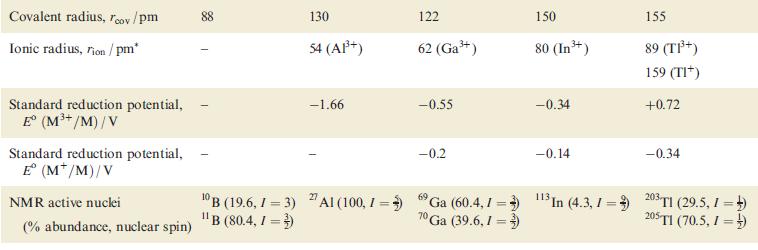
Property Atomic number, Z Ground state electronic configuration Enthalpy of atomization, AH(298 K)/kJ mol- Melting point, mp/K Boiling point, bp/K Standard enthalpy of fusion, Afus H (mp)/kJ mol First ionization energy, IE/kJ mol- Second ionization energy, IE/kJ mol- Third ionization energy, IE3/kJ mol- Fourth ionization energy, IE4/kJ mol- Metallic radius, metal/pm B 5 [He]2s2p 582 2453 4273 50.2 800.6 2427 3660 25 030 Al 13 [Ne]3s3p 330 933 2792 10.7 577.5 1817 2745 11 580 143 Ga 31 [Ar]3d04s4p 277 303 2477 5.6 578.8 1979 2963 6200 153 In 49 81 [Kr]4d05s5p [Xe]4f4 5d06s6p 243 430 2355 3.3 558.3 1821 2704 5200 167 182 576.5 1730 4.1 589.4 1971 2878 4900 171
Step by Step Solution
3.44 Rating (179 Votes )
There are 3 Steps involved in it
The given data from Table 131 includes the standard reduction potentials for various elements including thallium Specifically we are provided with the following standard reduction potentials Tl3 2e Tl ... View full answer

Get step-by-step solutions from verified subject matter experts


