Question: Using the data shown in the table, and given that the standard reduction potential for the Fe 3+ /Fe 2+ couple in aqueous solution is
Using the data shown in the table, and given that the standard reduction potential for the Fe3+/Fe2+ couple in aqueous solution is +0.77 V vs SHE, calculate the standard reduction potentials for the remaining redox couples. Compare your answers with values given in Resource section 3, and comment on your results.
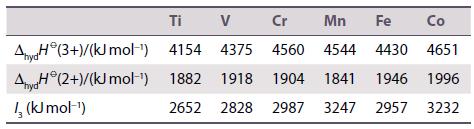
Co 4651 1841 1946 1996 2652 2828 2987 3247 2957 3232 Ti V Cr Mn Fe AnyH (3+)/(kJ mol-) 4154 4375 4560 4544 4430 Anya(2+)/(kJ mol-) 1882 1918 1904 I, (kJ mol-)
Step by Step Solution
3.43 Rating (162 Votes )
There are 3 Steps involved in it
To calculate the standard reduction potentials for the remaining redox couples we can use the Nernst equation Ecell Ecell RT nF lnQ Where Ecell is the ... View full answer

Get step-by-step solutions from verified subject matter experts


