Question: Given that for the path 1 2 3, a system absorbs 100 kJ as heat and does 60 kJ work. While along the
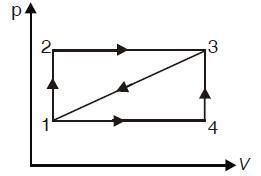
Given that for the path 1 – 2 – 3, a system absorbs 100 kJ as heat and does 60 kJ work.
While along the path 1 – 4 – 3, it does 20 kJ work (see figure). The heat absorbed during the cycle 1 – 4 – 3 is :
(a) – 140 kJ
(b) – 80 kJ
(c) – 40 kJ
(d) + 60 kJ
p. 2, 3 4
Step by Step Solution
3.37 Rating (156 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


