The half-life of a radioactive substance is the time it takes to decay by one-half. The half-life
Question:
The half-life of a radioactive substance is the time it takes to decay by one-half. The half-life of carbon 14, which is used for dating previously living things, is 5500 years. When an organism dies, it stops accumulating carbon 14. The carbon 14 present at the time of death decays with time. Let C(t)/C(0) be the fraction of carbon 14 remaining at time t. In radioactive carbon dating, scientists usually assume that the remaining fraction decays exponentially according to the following formula:
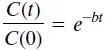
a. Use the half-life of carbon 14 to find the value of the parameter b, and plot the function.
b. If 90 percent of the original carbon 14 remains, estimate how long ago the organism died.
c. Suppose our estimate of b is off by ±1 percent. How does this error affect the age estimate?
Step by Step Answer:






