A reaction vessel is filled with a solution at 22.9C containing sulfuric acid and diethyl sulfate. The
Question:
A reaction vessel is filled with a solution at 22.9°C containing sulfuric acid and diethyl sulfate. The following reaction is first-order in each reactant and occurs with an irreversible reaction rate constant of 6.74×10−4 L/gmol s:
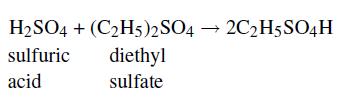
When the sulfuric acid concentration is 0.53 M and the diethyl sulfate is at 0.28 M, what reaction rate will be observed?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introduction To Chemical Engineering Tools For Today And Tomorrow
ISBN: 9780470885727
5th Edition
Authors: Kenneth A. Solen, John N. Harb
Question Posted:





