An object far denser than water can float if it is small enough and has the right
Question:
An object far denser than water can float if it is small enough and has the right surface properties. A result of surface tension, this is called capillary flotation. In what follows, suppose that each object is a cylinder of diameter D and length L, where L ≫ D.
(a) In which of the two situations in Fig. P4.11 might a dense cylinder (ρo > ρ) be able to float? Explain qualitatively.
(b) Derive an expression for the maximum upward force due to surface tension. When can end effects be neglected?
(c) Water striders are insects that routinely walk on water (Hu et al., 2003). A representative member of this family has six legs of 80 μm diameter, and when at rest on the surface the average length per leg that is in contact with water is 4 mm. For pond water, γ = 67 mN/m. Show that buoyancy is negligible relative to surface tension. What is the maximum mass m that this insect could have without sinking? Its actual mass is about 0.01 g.
(d) Although wood ordinarily floats, occasionally a log stored in a pond is denser than water and sinks to the bottom. Explain why surface tension could do little to help avoid such an inconvenience, even if the surface properties of the wood (contact angle) were optimal.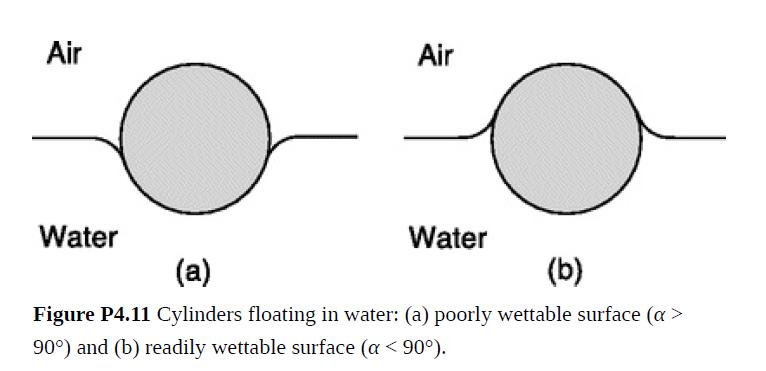
Step by Step Answer:

Introduction To Chemical Engineering Fluid Mechanics
ISBN: 9781107123779
1st Edition
Authors: William M. Deen





