Consider a mixture of species 1 and 2 in vaporliquid equilibrium at 25C and 90 bar. The
Question:
Consider a mixture of species 1 and 2 in vapor–liquid equilibrium at 25°C and 90 bar. The following equation of state is available for the vapor phase: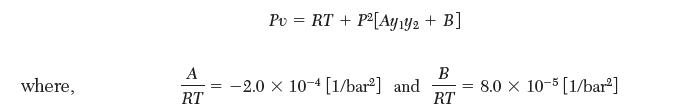
and y1 and y2 are the mole fractions of species 1 and 2, respectively. Species 2 is dilute in the liquid phase and may be described by Henry’s law with the following values at 25°C: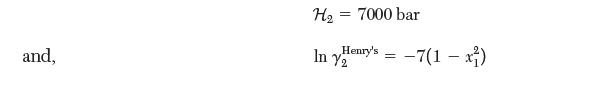
(a) Consider a vapor mixture with 5 mole of species 1 and 10 moles of species 2. Calculate the following quantities: ![]()
(b) Calculate an expression for the pure species fugacity coeffi cient, ![]() and the mixture fugacity coeffi cient,
and the mixture fugacity coeffi cient, ![]() of species 2 in the vapor.
of species 2 in the vapor.
(c) In the liquid, are like interactions stronger or weaker than unlike interactions? Explain.
(d) Find the mole fraction of species 2 in the liquid in equilibrium with the vapor in part (b).
(e) As best as you can, estimate the saturation pressure of pure species 2 at 25°C. State any assumptions that you make.
Step by Step Answer:






