Question: In Example 2.4, we solved a problem where 10.0 kg of water was reversibly compressed in a pistoncylinder assembly from a pressure of 20 bar
In Example 2.4, we solved a problem where 10.0 kg of water was reversibly compressed in a piston–cylinder assembly from a pressure of 20 bar and a volume of 1.0 m3 to a pressure of 100 bar.
In this example, the work was calculated to be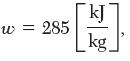
the heat was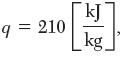
and the final temperature was T2 = 525°C.
Qualitatively answer the following questions. You do not have to do any calculations, but you must choose the right answer and explain your reasoning.
(a) Consider an adiabatic process that occurs from the same initial state (State 1) to the same final state (State 2). Will the magnitude of work required for the compression be (greater than, less than, or equal to) the value calculated in Example 2.4? Explain.
(b) Consider an isothermal process that occurs from the same initial state (State 1) to the same final pressure (P2). Will the heat transfer be greater than, less than, or equal to, the value calculated in Example 2.4? Explain.
(c) Consider an irreversible process that occurs from the same initial state (State 1) to the same final state (State 2). Will the magnitude of work required for the compression be greater than, less than, or equal to, the value calculated in Example 2.4? Explain.
(d) Consider an irreversible process that occurs from the same initial state (State 1) to the same final state (State 2). Will the heat transfer be greater than, less than, or equal to, the value calculated in Example 2.4? Explain.
w=285 kJ kg
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


