Question: In Section 2.3, we learned about reversible and irreversible processes in the context of a pistoncylinder assembly undergoing isothermal expansion and compression processes. Four of
In Section 2.3, we learned about reversible and irreversible processes in the context of a piston–cylinder assembly undergoing isothermal expansion and compression processes. Four of these processes are summarized in Figure 3.2:
(i) Irreversible expansion, process A
(ii) Irreversible compression, process B
(iii) Reversible expansion, processes E
(iv) Reversible compression, processes F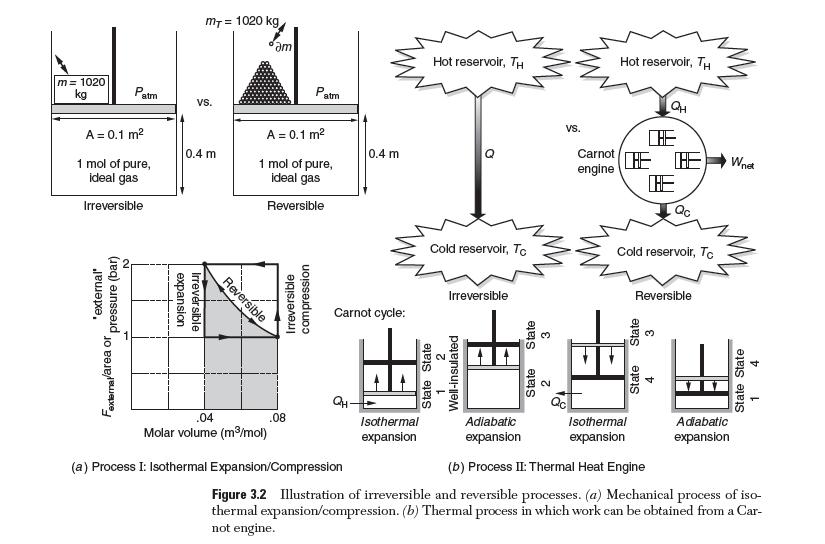
Calculate Δsuniv for each of these isothermal processes and show that the result is consistent with the second law of thermodynamics.
m= 1020 kg A = 0.1 m 1 mol of pure, ideal gas Irreversible 'external' pressure (bar) atm Fexternal/area or MT = 1020 kg VS. 0.4 m expansion Irreversible Reversible am .04 Molar volume (m/mol) A = 0.1 m 1 mol of pure, ideal gas Reversible compression Irreversible Pat .08 atm Carnot cycle: 0.4 m QH- (a) Process I: Isothermal Expansion/Compression Hot reservoir, TH Isothermal expansion Cold reservoir, To Irreversible vs. Adiabatic expansion Carnot engine Hot reservoir, TH 3881 QH CHE HE HE KE Cold reservoir, To Isothermal expansion Reversible (b) Process II: Thermal Heat Engine Adiabatic expansion Wnet 4 State State 1 Figure 3.2 Illustration of irreversible and reversible processes. (a) Mechanical process of iso- thermal expansion/compression. (b) Thermal process in which work can be obtained from a Car- not engine.
Step by Step Solution
3.35 Rating (167 Votes )
There are 3 Steps involved in it
In this example we must consider both the entropy change of the system and the entropy change of the surroundings We outline the solution methodology with process A the isothermal irreversible expansi... View full answer

Get step-by-step solutions from verified subject matter experts


