You wish to calculate in a mixture of 25.0 mole % propylene (1) and 75.0 mole %
Question:
You wish to calculate in a mixture of 25.0 mole % propylene (1) and 75.0 mole % methane (2) at 219.0 K and 27.72 bar. For all your answers, use the following equation of state: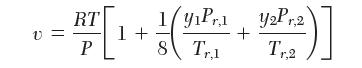
where the reduced pressure and temperature are used: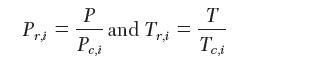
(a) i. Develop expressions for the fugacity and the fugacity coeffi cient of propylene using the Lewis fugacity rule.
ii. Write the expression for the fugacity coeffi cient in reduced coordinates.
iii. Calculate the values for the fugacity and the fugacity coeffi cient of propylene.
(b) i. Develop expressions for the fugacity and the fugacity coeffi cient of propylene using the complete equation of state method for mixtures.
ii. Calculate the values for the fugacity and the fugacity coeffi cient of propylene.
(c) Compare your results from Parts
(a) and (b). How do you explain the results?
Step by Step Answer:






