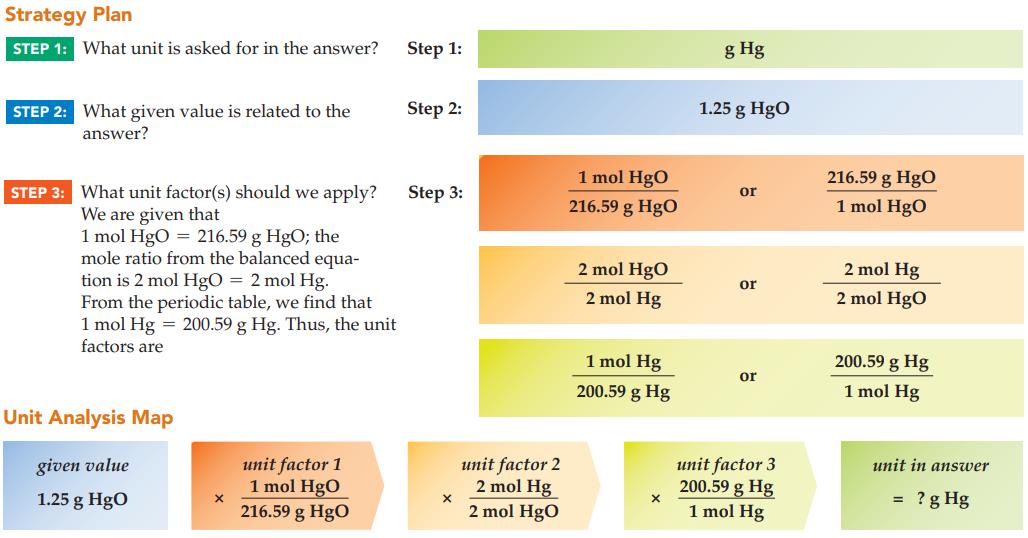
Calculate the mass of mercury produced from the decomposition of 1.25 g of orange mercury(II) oxide (MM
Question:
Calculate the mass of mercury produced from the decomposition of 1.25 g of orange mercury(II) oxide (MM = 216.59 g/mol):
![]()
Transcribed Image Text:
2 HgO(s) A 2 Hg(1) + O₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
We select from each of the three pai...View the full answer

Answered By

Arjun chauhan
I have gained more knowledge in area of Information & Technology. Although i am still learning much more.
I find computer science much fascinate to work on.
My most area of interest of work belong to DATABASE, database language like SQL & PL/SQL, cloud technology & system architecture ,Networking etc.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
Which is true regarding the bottom-up approach to budgeting expected project costs? A. The bottom-up approach allocates the overarching budget across the work packages. B. The bottom-up approach...
-
Calculate the mass of octane, C8H18(l), that must be burned in air to evolve the same quantity of energy as produced by the fusion of 1.0 g of hydrogen in the following fusion reaction: Assume that...
-
What line of code can you add to disable all logging messages in your program?
-
Why would the use of the cash payback period for analyzing the financial performance of theatrical releases from a motion picture production studio be supported over the net present value method?
-
A company has 10,000 employees. Each employee is entitled to twenty days of paid holiday per calendar year. Up to five days of this entitlement may be carried forward and taken in the following year...
-
Is the collision in Example 10.7 elastic? Data from Example 10.7 Pucks 1 and 2 slide on ice and collide. The inertia of puck 2 is twice that of puck 1 . Puck 1 initially moves at \(1.8 \mathrm{~m} /...
-
At the beginning of 2014, Salinas Company incurred the following start-up and organization costs: (1) attorneys fees with a market value of $10,000, paid with 6,000 shares of $1 par value common...
-
11.A simple LR circuit is connected to a battery at t = 0. The time instant at which rate of energy storage in inductor is half of power delivered by battery 2L (1) In 2 (3) In 2 (2) In (4) (4) In 3
-
What are the three steps in the unit analysis method of problem solving?
-
Classify the following type of stoichiometry problem: How many cubic centimeters of oxygen are produced from decomposing 5.00 mL of steam? (a) Massmass problem (b) Massvolume problem (c) Volumevolume...
-
What are the two requirements that must be satisfied to perform a goodness-of-fit test?
-
should we still go for social media messaging as part of the business overall strategy and what are mitigations to the risks? Explain
-
To finance the development of a new product, a company borrowed $28,000 at 3% compounded monthly. If the loan is to be repaid in equal semi-annually payments over ten years and the first payment is...
-
describe the prototypical career progression of a person in the homeland security field, from the junior to senior levels. What are the expectations related to each level with respect to job...
-
Think of the problem of climate change as a "game" between two countries called Brazil and Germany, considered as if each were a single individual. Each country has two possible strategies for...
-
Consider the following information describes the expected return and risk relationship for three stocks: Lasco Foods Grace Manufacturing Wysinco Ltd. Market Risk Premium Risk Free Rate Expected...
-
In the mid-1990s, a large consumer goods manufacturer moved its customer-based department and specialty stores to mass merchandising in a a variety of retail stores, large and small. The strategic...
-
From 1970 to 1990, Sri Lanka's population grew by approximately 2.2 million persons every five years. The population in 1970 was 12.2 million people.What is the best formula for P, Sri Lanka's...
-
Name the five steps in process costing when equivalent units are computed?
-
Name the three inventory methods commonly associated with process costing.
-
Describe the distinctive characteristic of weighted-average computations in assigning costs to units completed and to units in ending work in process.
-
Develop a presentation summarizing your analysis of the assigned Code provision and address the following questions: What ethical theories or principles is this provision designed to uphold? What...
-
Marina Company Work Sheet For the Year Ended December 31, 2012 Trial Balance Adjustments Income Statement Balance Sheet Accounts Cash Dr. Cr. Ref. Dr. Cr. Ref. Dr. Cr. Ref. Dr. Cr. S 12.400 Equity...
-
In light of the recent interest rate hikes by the Federal Reserve to fight inflation, economists and financial market experts are debating the effectiveness of a tight monetary policy on inflation...

Study smarter with the SolutionInn App


