Given the three-step blast furnace process that converts iron ore, Fe 2 O 3 , into molten
Question:
Given the three-step “blast furnace” process that converts iron ore, Fe2O3, into molten “pig iron":
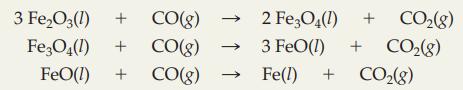
What mass of Fe2O3 produces 1.00 metric ton (1000 kg) of iron?
(a) 175 kg
(b) 699 kg
(c) 715 kg
(d) 1430 kg
(e) 2860 kg.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:





