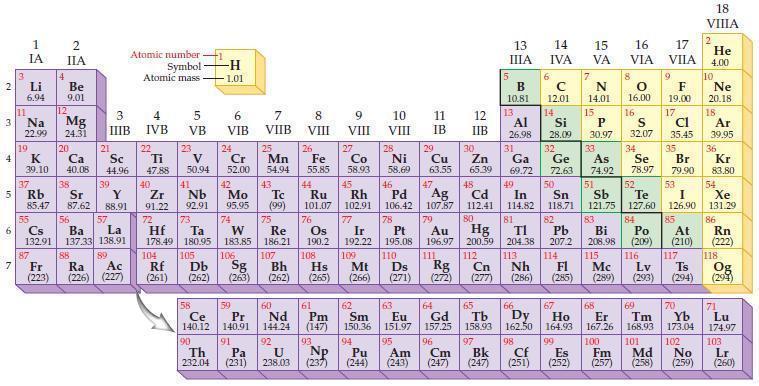
Refer to the periodic table and state the noble gas with an electron configuration identical to each
Question:
Refer to the periodic table and state the noble gas with an electron configuration identical to each of the following ions.
(a) S2–
(b) Cl–
(c) K+
(d) Ca2+.
Periodic Table
Transcribed Image Text:
2 3 4 5₁ 6 7 3 11 Li 6.94 1 IA Na 22.99 19 37 al 55 4 87 2 IIA Fr (223) Be 9.01 12 K Ca Sc 39.10 40.08 44.96 Mg 24.31 38 Rb Sr Y 85.47 87.62 88.91 56 57 Cs Ba La 132.91 137.33 138.91 20 21 88 3 IIIB 39 89 Atomic number Symbol - Ac Ra (226) (227) Atomic mass 4 IVB 22 Ti 47.88 40 5 VB 23 V 50.94 41 Zr Nb 91.22 92.91 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 72 73 W Re Hf Ta 178.49 180.95 183.85 186.21 91 7 VIIB Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 108 9 VIII 61 Pm (147) 27 Bh Hs Mt (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB Pt 195.08 110 29 Cu 63.55 13 IIIA 12 IIB 5 B 10.81 13 17 16 VA VIA VIIA 8 Al 26.98 6 C 12.01 14 Si 28.09 32 30 31 33 As Se Br Zn Ga Ge 65.39 69.72 72.63 74.92 78.97 79.90 14 15 IVA 7 N 14.01 15 P 30.97 16.00 83 Bi 208.98 115 16 S 32.07 34 84 47 48 49 50 51 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 79 Au Hg 196.97 200.59 111 112 Rg Cn (271) (272) (277) (286) (285) (289) (293) (294) 81 82 TI Pb 204.38 207.2 113 114 Nh Fl Ds Mc Lv Ts Po (209) 9 116 F 19.00 Md (258) 17 Cl 35.45 35 53 I 126.90 85 At (210) 66 67 69 70 63 64 65 68 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 98 99 100 Cf Es Fm (251) (252) (257) 117 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Noble Gas for Each Ion a S2 Neon Ne Sulfur has 16 electronsand when gai...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and state the noble gas with an electron configuration identical to each of the following ions? (a) Se 2 (b) Br (c) Rb + (d) Sr 2+ . Periodic Table 2 3 4 5 6 7 3 11 Li...
-
Refer to the periodic table and write the predicted electron configuration for each of the following negative ions using core notation. (a) Br (b) Te 2 (c) As 3 (d) O 2 . Periodic Table: 2 3 4 10...
-
Refer to the periodic table and write the predicted electron configuration for each of the following negative ions using core notation. (a) F (b) S 2 (c) N 3 (d) I . Periodic Table: 2 3 4 10 6 3...
-
Marks 1. Find the limits, if they exist. If a limit does not exist, check whether the function approaches +00 x2 + 2x - 15 (5) (a) lim x-3 x2-4x +3 x2 - 4 (5) (b) lim x2 x4 - 16 Carol Ferland CF...
-
A firm emerging from Chapter 11 bankruptcy that does not qualify for fresh-start reporting must still report the effect of the reorganization plan on its financial position and results of operations....
-
Prove Equation 10.33, starting with Equation 10.32. i (20 + 1) [je(ka) + ik ach" (ka)] Pe(cos ) : = 0 8=0 (10.32)
-
Your firm uses a large parallel-plate capacitor to store energy, and you measure the electric field strength between the plates to determine the amount of energy stored. During a test run with a new...
-
Compute the specified ratios using Bryce Companys balance sheet at December 31, 2008. Assets Cash ............... $ 18,000 Marketable securities ......... 8,000 Accounts receivable ......... 13,000...
-
The definition, " ' Chiropractor ' means a person who is a medical quack with no legitimate scientific background," is an example of: Theoretical definition Definition by subclass Persuasive...
-
Using VSEPR theory, contrast the molecular shape of an ammonia molecule, NH3 , with that of an ammonium ion, NH4+.
-
Write out the electron configuration for each of the following nonmetal ions. (a) F (b) O 2 (c) N 3 (d) C 4 .
-
1 and 2 are supplementary angles. Given the measure of 1, find m2. m/1 = 155
-
Suppose that A is the set {a, b, c, d} and that R is a relation defined on set A such that R is represented by the digraph shown below. Furthermore, suppose that S is the reflexive closure of R. What...
-
Assuming Jay and JoAnn have this same business that they run as a partnership, prepare their joint Federal income tax return for 2022. Attached is the copy of the partnership K-1 for each of them....
-
6. The conversion of nitrogen dioxide gas into dinitrogen tetroxide gas follows simple second order kinetics. a. Calculate the rate constant if the rate of the reaction is 8.49 M/sec when the...
-
4- Consider an airplane. For this airplane the zero-lift angle of attack is -1.5, the lift slope (ao) of the airfoil section is 0.112 per degree, the lift efficiency factor t = 0.04, and the wing...
-
Regression equation for Case 3.0: SUMMARY OUTPUT Regression Statistics Multiple R 0.957 R Square 0.915 Adjusted R Square 0.908 Standard Error 5.779 Observations 52 ANOVA df SS MS F Significance F...
-
In 2010, Nuts & Seeds Inc., purchased a new high-tech shelling machine from Soft-Core Corporation. Nuts and Seeds paid $1,000 in cash and gave Soft-Core a $29,000 note. The note is non-recourse and...
-
Two mutually exclusive investment alternatives are being considered. Alternative A requires an initial investment of $20,000 in a machine. Annual operating and maintenance costs are anticipated to be...
-
Classification at costs, merchandising sector Home Entertainment Center (HEC) operates a large store in San Francisco. The store has both a video section and a music (compact disks and tapes)...
-
Classification of costs, manufacturing sector, the Fremont, California, plant of New United Motor Manufacturing, Inc. (NUMMI), a joint venture of General Motors and Toyota, assembles two types of...
-
Variable costs, fixed costs, total costs. Ana Compo is getting ready to open a small restaurant. She is on a tight budget and must choose between the following long-distance phone plans: Plan A: Pay...
-
Fasharel is an innovative company selling casual, yet fashionable, apparel. It purchases the clothing from a manufacturer and sells directly to consumers through its website. The dataset Orders.xlsx...
-
Gregor Olson works at the National Bank branch in Copenhagen. According to the banks records, there is currently an outstanding loan of $380,000 to Danville Bus Services, a company that schedules...
-
1 . Briefly deliver a short individual presentation on methods by which feedback can be elicited, analysed and interpreted. Content: Introduction, Preview of the presentation, informing the audience...

Study smarter with the SolutionInn App


