Question: Without referring to Table 11.6, predict which compound in each of the following pairs has the higher boiling point: (a) H 2 O or H
Without referring to Table 11.6, predict which compound in each of the following pairs has the higher boiling point:
(a) H2O or H2Se
(b) H2S or H2Te.
Table 11.6
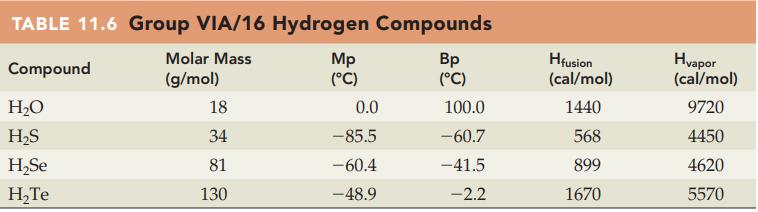
TABLE 11.6 Group VIA/16 Hydrogen Compounds Molar Mass Compound (g/mol) HO HS HSe HTe 18 34 81 130 Mp (C) 0.0 -85.5 -60.4 -48.9 Bp (C) 100.0 -60.7 -41.5 -2.2 Hfusion (cal/mol) 1440 568 899 1670 Hvapor (cal/mol) 9720 4450 4620 5570
Step by Step Solution
3.35 Rating (158 Votes )
There are 3 Steps involved in it
a H2O vs H2Se Atomic number Oxygen O has a lower atomic number 8 compared to selenium Se with 34 Gen... View full answer

Get step-by-step solutions from verified subject matter experts


