The conversion of a second-order reaction in a tubular reactor with axial dispersion, can be overestimated by
Question:
The conversion of a second-order reaction in a tubular reactor with axial dispersion,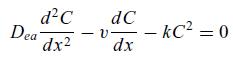
can be overestimated by replacing the second-order reaction by a first-order reaction using the maximal concentration in the reactor,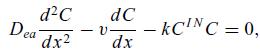
where CIN is the inlet concentration. A better approximation is obtained if CIN is replaced by the average concentration, calculated from the solution of the first simple approximation with CIN . Using the average concentration will underestimate the conversion in the first part of the reactor by giving a reaction rate at the inlet that is too low and consequently overestimating the reaction rate in the rear part.
Step by Step Answer:

Mathematical Modeling In Chemical Engineering
ISBN: 9781107049697
1st Edition
Authors: Anders Rasmuson, Bengt Andersson, Louise Olsson, Ronnie Andersson





