If a hydrogen atom is in a 2s state, the probability of finding the electron at a
Question:
If a hydrogen atom is in a 2s state, the probability of finding the electron at a distance r from the nucleus is proportional to 4Πr2Ψ22swhere Ψ represents the orbital (wave function):
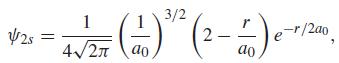
where a0 is a constant known as the Bohr radius, equal to 0.529 × 10-10m.
(a) Locate the maxima and minima of Ψ2s.
(b) Draw a rough graph of Ψ2s.
(c) Locate the maxima and minima of Ψ22s.
(d) Draw a rough graph of Ψ22s.
(e) Locate the maxima and minima of 4Πr2Ψ22s.
(f) Draw a rough graph of 4Πr2Ψ22s.
Transcribed Image Text:
1 3/2 25 2- e/2a0 4/27 (ao do
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
a To find the extrema we omit the constant factor We cancel the exponential factor which is the same ...View the full answer

Answered By

Akshay Singla
as a qualified engineering expert i am able to offer you my extensive knowledge with real solutions in regards to planning and practices in this field. i am able to assist you from the beginning of your projects, quizzes, exams, reports, etc. i provide detailed and accurate solutions.
i have solved many difficult problems and their results are extremely good and satisfactory.
i am an expert who can provide assistance in task of all topics from basic level to advance research level. i am working as a part time lecturer at university level in renowned institute. i usually design the coursework in my specified topics. i have an experience of more than 5 years in research.
i have been awarded with the state awards in doing research in the fields of science and technology.
recently i have built the prototype of a plane which is carefully made after analyzing all the laws and principles involved in flying and its function.
1. bachelor of technology in mechanical engineering from indian institute of technology (iit)
2. award of excellence in completing course in autocad, engineering drawing, report writing, etc
4.70+
48+ Reviews
56+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Mathematics questions
-
(a) A water molecule consists of two hydrogen atoms connected at an angle of 105o to an oxygen atom whose relative mass is 16 times that of the hydrogen atoms. If the bonds are modeled as linear unit...
-
In a certain city, 8 percent of the cars have a burned-out headlight. (a) What is the expected number that must be inspected before the first one with a burned-out headlight is found? (b) What is the...
-
On average, there are 12 potholes per mile on a particular stretch of the state highway. Suppose the potholes are distributed evenly on the highway. a. Find the probability of finding fewer than two...
-
Tell whether the given side lengths form a right triangle. 8, 10, and 14
-
The mixing department manager of Crede Company is able to control all overhead costs except rent, property taxes, and salaries. Budgeted monthly overhead costs for the mixing department, in...
-
You have been assigned to the first audit of the Chicago Company for the year ending March 31, 2019. Accounts receivable were confirmed on December 31, 2018, and at that date the receivables...
-
Describe how those who contract AIDS are discriminated against.
-
Johson Corporation issued bonds twice during 2010. The transactions were as follows: 2010 Jan 1Issued $1,000,000 of 7.5 percent, 10-year bonds dated January 1, 2010, with interest payable on June 30...
-
5. Add a worksheet labeled 'Pivot Chart' and move it so that it's the first one on the left. Then create a Pivot Chart (Column Chart) showing Country Name and Sum of USD Total. Format all cells...
-
You have been asked to review how well your company is prepared for a major data breach of your firms customer database containing some 15 million records with names, addresses, passwords, credit...
-
Find the maximum and minimum values of the function y = x 3 + 4x 2 10x in the interval 5 < x < 5.
-
According to the Planck theory of black-body radiation, the radiant spectral emittance is given by the formula where is the wavelength of the radiation, h is Plancks constant, k B is Boltzmanns...
-
Determine the sum of the arithmetic sequence. The number of terms, n, is given. 0.5, 0.75, 1.00, 1.25, . . ., 5.25; n=20
-
Which of the following values are not probabilities? 5:2 3/7 7/3 -0.9 0.123 123/456 456/123 0 1
-
When guessing the answer to a multiple-choice question on an SAT test, the chance of guessing correctly is 1 in 5. Express the indicated degree of likelihood as a probability value between 0 and 1.
-
Given that the following statement is incorrect, rewrite it correctly: The probability of a baby being born a boy is 5050.
-
Based on a Harris poll, there is a 5050 chance that a randomly selected adult has pierced ears. Express the indicated degree of likelihood as a probability value between 0 and 1.
-
Using the information in this chapter, determine which tax form you should use and outline the types of records you will need. Talk with several taxpayers in different socioeconomic groups or stages...
-
Consider the following computer output for a 23 factorial experiment. (a) How many replicates were used in the experiment? (b) Use the appropriate equation to calculate the standard error of a...
-
The Ranch 888 Noodle Company sells two types of dried noodles:ramen, at $6.50 per box, and chow fun, at $7.70 per box. So farthis year, the company has sold a total of 110,096 boxes ofnoodles,...
-
Gallup Organization Date: May 2016 Population: American adults Question: Describe your views on social issues. Responses: 1. Liberal 2. Moderate 3. Conservative a. Determine the frequency and the...
-
Gallup Organization Date: May 2016 Population: American adults Question: Describe your views on economic issues. Responses: 1. Liberal 2. Moderate 3. Conservative a. Determine the frequency and the...
-
Has the educational level of adults changed over the past 15 years? To help answer this question the Bureau of Labor Statistics compiled the following table, which lists the number (1,000) of adults...
-
sked byChina699 BEMIDJI STATE UNIVERSITY Department of Technology, Art & Design TADT 3217 : Materials Science & Metallurgy Hardness Testing [A continued look at the Heat Treatment of Steel] ...
-
1. Advocate Aurora Sheboygan Memorial Hospital health care product or service to be marketed in your community. 2. Conduct appropriate market research in your community to determine the demographics...
-
How do ethical leaders integrate ethical considerations into strategic decision-making processes, balancing short-term business objectives with long-term ethical imperatives to ensure sustainable...

Study smarter with the SolutionInn App


