Question: The entropy change to bring a sample from 0 K (absolute zero) to a given state is called the absolute entropy of the sample in
The entropy change to bring a sample from 0 K (absolute zero) to a given state is called the absolute entropy of the sample in that state.
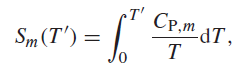
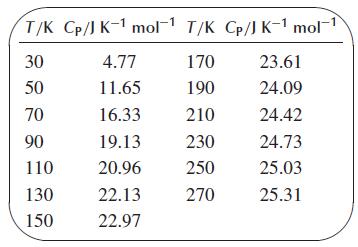
where Sm(T′) is the absolute molar entropy at temperature T′,CP,m is the molar heat capacity at constant pressure, and T is the absolute temperature. Using Simpson’s rule, calculate the absolute entropy of 1.000 mol of solid silver at 270 K. For the region 0–30 K, use the approximate relation Cp = aT3, where a is a constant that you can evaluate from the value of Cp at 30 K. For the region 30–270 K, use the following data:3
" , -dT, Sm (T') =
Step by Step Solution
3.28 Rating (172 Votes )
There are 3 Steps involved in it
We divide the integral into two parts one from t 0 K to T 30 K ... View full answer

Get step-by-step solutions from verified subject matter experts


