The van der Waals equation of state is For carbon dioxide, a = 0.3640 Pa m 6
Question:
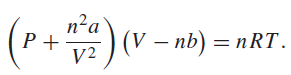
For carbon dioxide, a = 0.3640 Pa m6 molˆ’1 and b = 4.267 × 10ˆ’5 m3 molˆ’1. Find the pressure of 0.7500 mol of carbon dioxide if V = 0.0242 m3 and T = 298.0 K. Find the uncertainty in the pressure if the uncertainty in the volume is 0.00004 m3 and the uncertainty in the temperature is 0.5 K. Assume that the uncertainty in n is negligible. Find the pressure predicted by the ideal gas equation of state. Compare the difference between the two pressures you calculated and the expected error in the pressure.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





