The van der Waals equation of state is When the temperature of a given gas is equal
Question:
The van der Waals equation of state is
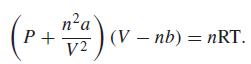
When the temperature of a given gas is equal to its critical temperature, the gas has a state at which the pressure as a function of V at constant T and n exhibits an inflection point at which dP/dV = 0 and d2P/dV2 = 0. This inflection point corresponds to the critical point of the gas. Write P as a function of T, V, and n and write expressions for dP/dV and d2P/dV2, treating T and n as constants. Set these two expressions equal to zero and solve the simultaneous equations to find an expression for the pressure at the critical point.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





