A DNA molecule consists of two long strands wound around each other as a helix, forming a
Question:
A DNA molecule consists of two long strands wound around each other as a helix, forming a cylinder with radius a ≈ 1nm. In this exercise, we explore three ways of measuring the molecule’s Young’s modulus E. For background and further details, see Marko and Cocco (2003) and Nelson (2008, Chap. 9).
(a) Show that if a segment of DNA with length ℓ is bent into a segment of a circle with radius R, its elastic energy is Eel = Dℓ/(2R2), where D = (π/4)a4E is the molecule’s flexural rigidity.
(b) Biophysicists define the DNA’s persistence length ℓp as that length which, when bent through an angle of 90◦, has elastic energy Eel = kBT , where kB is Boltzmann’s constant and T is the temperature of the molecule’s environment. Show that ℓp ≈ D/(kBT). Explain why, in a thermalized environment, segments much shorter than ℓp will be more or less straight, and segments with length ∼ℓp will be randomly bent through angles of order 90◦.
(c) Explain why a DNA molecule with total length L will usually be found in a random coil with diameter
![]()
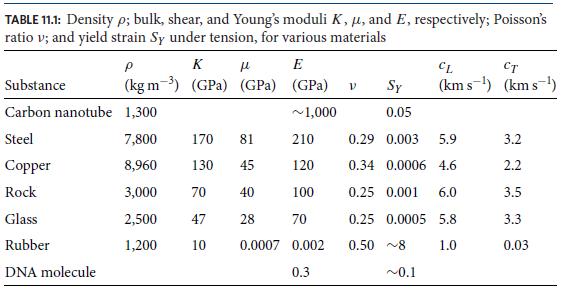
Observations at room temperature with L ≈ 17 μm reveal that d ≈ 1μm. From this show that the persistence length is ℓp ≈ 50 nm at room temperature, and thence evaluate the molecule’s flexural rigidity and from it, show that the molecule’s Young’s modulus is E ≈ 0.3GPa; cf. Table 11.1.
(d) When the ends of a DNA molecule are attached to glass beads and the beads are pulled apart with a gradually increasing force F, the molecule begins to uncoil, just like rubber. To understand this semi quantitatively, think of the molecule as like a chain made of N links, each with length ℓp, whose interfaces can bend freely. If the force acts along the z direction, explain why the probability that any chosen link will make an angle θ to the z axis is dP/d cos θ ∝ exp[+Fℓp cos θ/(kBT)].
Infer that when the force is F, the molecule’s length along the force’s direction is L̅ ≈ L(coth α − 1/α), where α = Fℓp/(kBT) and L = Nℓp is the length of the uncoiled molecule. Infer, further, that for α «1 (i.e., F « kBT/ℓp 0.1 pN), our model predicts L̅ ≈ αL/3, i.e. a linear force-length relation F = (3kBT/ℓp) L̅/L, with a strongly temperature dependent spring constant, 3kBT/ℓp ∝ T2. The measured value of this spring constant, at room temperature, is about 0.13 pN. From this infer a value 0.5 GPa for the molecule’s Young’s modulus. This agrees surprisingly well with the 0.3 GPa deduced in part (c), given the crudeness of the jointed chain model.
(e) Show that when F » kBT/ℓp ∼ 0.1pN, our crude model predicts (correctly) that the molecule is stretched to its full length L = Nℓp. At this point, its true elasticity should take over and allow genuine stretching. That true elasticity turns out to dominate only for forces
![]()
For a force between ∼10 and ∼80pN, the molecule is measured to obey Hooke’s law, with a Young’s modulus E ≈ 0.3GPa that agrees with the value inferred in part (c) from its random-coil diameter. When the applied force reaches ≈ 65 pN, the molecule’s double helix suddenly stretches greatly with small increases of force, changing its structure, so this is the molecule’s yield point. Show that the strain at this yield point is △ℓ/ℓ ∼ 0.1; cf. Table 11.1.
Table 11.1
Step by Step Answer:

Modern Classical Physics Optics Fluids Plasmas Elasticity Relativity And Statistical Physics
ISBN: 9780691159027
1st Edition
Authors: Kip S. Thorne, Roger D. Blandford





