Question: Molecules adsorbed on the surface of a solid crystal surface at low temperature typically arrange themselves into a periodic arrangement, e.g., one molecule lies at
Molecules adsorbed on the surface of a solid crystal surface at low temperature typically arrange themselves into a periodic arrangement, e.g., one molecule lies at the center of each a × a square of a two-dimensional checkerboard formed by the surface atoms of the crystal. For diatomic molecules which adsorb with their long axis parallel to the surface, the orientation of each molecule is determined by the lowest-order electrostatic interaction between nearby molecules.
(a) The CO molecule has a small electric dipole moment p. The sketch below shows a portion of the complete checkerboard where the arrangement of dipole moments is parameterized by an angle α. Treat these as point dipoles and consider the interaction of each dipole with its eight nearest neighbors only. Find the angle α that minimizes the total energy and show that the energy/dipole is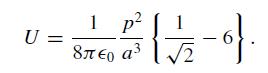
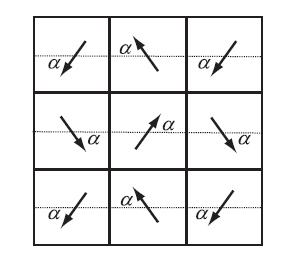
(b) The N2 molecule has a small quadrupole moment because covalent bonding builds up some negative (electron) charge in the bond region between the atomic nuclei. Use this fact and a qualitative argument to help you make a sketch which illustrates the orientational order for a checkerboard of N2 molecules
U= = Race (1/1/2-9). 1 p a a
Step by Step Solution
3.46 Rating (159 Votes )
There are 3 Steps involved in it
a The interaction energy between a point dipole p 1 at r 1 and a point dipole p ... View full answer

Get step-by-step solutions from verified subject matter experts


