A chemical dissolves in water at a rate jointly proportional to the amount undissolved and to the
Question:
A chemical dissolves in water at a rate jointly proportional to the amount undissolved and to the difference between the concentration in the solution and that in the saturated solution. Initially none of the chemical is dissolved in the water. Show that the amount x(t) of undissolved chemical satisfies the differential equation
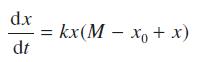
where k is a constant, M is the amount of the chemical in the saturated solution and x0 = x(0).
Transcribed Image Text:
dx dt = kx(M - xo + x)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Amount undissolved is x at time t Amount i...View the full answer

Answered By

Hemstone Ouma
"Hi there! My name is Hemstone Ouma and I am a computer scientist with a strong background in hands-on experience skills such as programming, sofware development and testing to name just a few. I have a degree in computer science from Dedan Kimathi University of Technology and a Masters degree from the University of Nairobi in Business Education. I have spent the past 6 years working in the field, gaining a wide range of skills and knowledge. In my current role as a programmer, I have had the opportunity to work on a variety of projects and have developed a strong understanding of several programming languages such as python, java, C++, C# and Javascript.
In addition to my professional experience, I also have a passion for teaching and helping others to learn. I have experience as a tutor, both in a formal setting and on a one-on-one basis, and have a proven track record of helping students to succeed. I believe that with the right guidance and support, anyone can learn and excel in computer science.
I am excited to bring my skills and experience to a new opportunity and am always looking for ways to make an impact and grow as a professional. I am confident that my hands-on experience as a computer scientist and tutor make me a strong candidate for any role and I am excited to see where my career will take me next.
5.00+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A curved bar ABC is subjected to loads in the form of two equal and opposite forces P, as shown in the figure. The axis of the bar forms a semicircle of radius r. Determine the axial force N, shear...
-
In the circuit shown in Fig all the resistors are rated at a maximum power of 1.00 W. What is the maximum emf e that the battery can have without burning up any of the resistors? 25.0 1 25.0n 30.00...
-
Suppose the time between the arrivals of successive cars at a toll booth is measured by a random variable X that is exponentially distributed with the density function where x measures time (in...
-
In Exercises show that the function y = (x) is a solution of the differential equation. y = 4e-x y" - y = 0
-
A beam of hydrogen molecules (H2) is directed toward a wall, at an angle of 55o with the normal to the wall. Each molecule in the beam has a speed of 1.0 km/s and a mass of 3.3 x 10-24 g. The beam...
-
Using the y-x and T-y-x diagrams in Figures 4.3 and 4.4, determine the temperature, amounts, and compositions of the equilibrium vapor and liquid phases at 101 kPa for the following conditions with a...
-
0.993 Use the Standard Normal Table or technology to find the z-score that corresponds to the cumulative area or percentile. Table 4-Standard Normal Distribution Arca z 0 z Z .09 .08 .07 .06 .05 .04...
-
A survey of MBA students obtained the following data on Students first reason for application to the school in which they matriculated. a. Develop a joint probability table using these data. b. Use...
-
10.) How many iterations will the following loop execute? int intIndex = 100; while (int Index < 10) Console.WriteLine ("hello"); int Index += 1;
-
The rate at which a solute diffuses through a membrane is proportional to the area and to the concentration difference across the membrane. A solution of concentration C flows down a tube with...
-
The limiting tension in a rope wound round a capstan (that is, the tension when the rope is about to slip) depends on the angle of wrap , as shown in Figure 8.15. Show that an increase in the angle...
-
Write concurrent TCP client-server programs to simulate a simplified version of POP. The client sends a request to receive an e-mail in its mailbox; the server responds with the e-mail.
-
Imagine that there are two stock markets in the world, whose relative capitalization weights are \(25 \%\) and \(75 \%\), respectively. The expected returns of the two markets are \(6 \%\) and \(4...
-
You are analyzing three stock shares: Joint, Eppon, and Peculiar Motors. Based on your analysis, the price of a Joint stock share should be the same as the sum of one Eppon share and one Peculiar...
-
A stock price is currently \(\$ 40\). It is known that, in one month, the price will be either \(\$ 42\) or \(\$ 38\). The annual risk-free interest rate is \(8 \%\), with continuous compounding....
-
Consider an American-style put option written on a stock share that does not pay dividends. The continuously compounded risk-free rate is 3%. The option matures in nine months and its strike is...
-
You are a German investor who enters into a futures contract to buy \(150,000 \mathrm{GBP}\) in four months. When you take this long position, the following data apply: Spot price of one GBP is \(...
-
Brenda Wong is a licensed real estate broker specializing in vacation homes and investment properties in the Sedona, Arizona area. Because of her affiliation with a large national real estate agency...
-
Draw the form of l-glutamic acid that predominates at each pH: (a) 1.9 (b) 2.4 (c) 5.8 (d) 10.4
-
For each of the following amino acids, draw the form that is expected to predominate at physiological pH: (a) l-Isoleucine (b) l-Tryptophan (c) l-Glutamine (d) l-Glutamic acid
-
Using the data in the following table, calculate the pI of the following amino acids: (a) l-Alanine (b) l-Asparagine (c) l-Histidine (d) l-Glutamic acid THE PK,VALUES FOR TWENTY NATURALLY OCCURRING...
-
Hatch Inc. has the following transactions during March 2027: Transaction 1: Mar. 1 The business received $10,000 cash and issued common stock to stockholders. Transaction 2: Mar. 2 Paid the rent of...
-
Compute the missing amount in the accounting equation for each entity from the financial information presented: Assets Liabilities Equity Style Cuts $? $ 37,000 $ 37,000 Hair Styles 71,000 ? 33,000...
-
MAP 2.4 Tesco Locations and Its Cross-Cultural Project The orange-colored countries on the map show Tesco's operations by country as of 2015.The arrows indicate the countries sending personnel to the...

Study smarter with the SolutionInn App


