(a) Calculate the electrostatic potential energy of Na + and Cl - ions at their equilibrium separation...
Question:
(a) Calculate the electrostatic potential energy of Na+ and Cl- ions at their equilibrium separation distance of 0.24 nm, assuming the ions to be point charges.
(b) What is the energy of repulsion at this separation?
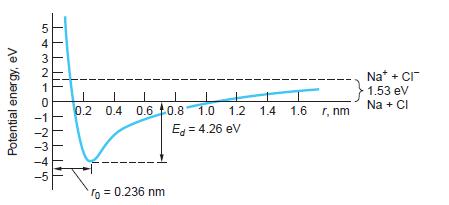
(c) Assume that the energy of repulsion is given by Equation 9-2. From Figure 9-2b, this energy equals ke2/r at about r = 0.14 nm. Use this and your answer to part (b) to calculate n and A. (Although this calculation is not very accurate, the energy of repulsion does vary much more rapidly with r than does the energy of attraction.)
Equation 9-2

Figure 9-2b
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





